I’ve talked a lot on this blog about how understanding the quantum states of a material can be helpful for working out its properties. But is it possible to directly measure these states in an experiment? And what sort of equipment is needed to do so? I’ll try to explain here.
First, a quick recap. The band structure is like a map of the allowed quantum states for the electrons in a material. The coordinates of the map are the momentum of the electron, and at each point there are a series of energy levels which the electron can be in. The energy states close to the “Fermi energy” largely determine things like whether the material can conduct electricity and heat, absorb light, or do interesting magnetic things.
There are various ways that the band structure can be investigated. Some of them are quite indirect, but last week, I visited an experimental facility in the UK where they can do (almost) direct measurements of the band structure using X-rays.
The technical name for this technique is “angle-resolved photoemission spectroscopy”, or ARPES for short. Let’s break that down a bit. Spectroscopy just means that it’s a way of measuring the spectrum of something. In this case, it’s the electrons in the material. I’ll come back to the “angle-resolved” part in a minute, but the crucial thing to explain here is what photoemission is.

The sketch above shows a hypothetical band structure. When light is shone on a material, the photons (green wavy arrows) that make up the beam can be absorbed by one of the electrons in the filled bands below the Fermi energy. When this happens, the energy and momentum of the photon is transferred into the electron.
This means that the electron must change its quantum state. But the band structure gives the map of the only allowed states in the material, so the electron must end up in one of the other bands. In the left-hand picture, the energy of the photon is just right for the electron at the bottom of the red arrow to jump to an unfilled state above the Fermi energy. This is called “excitation”.
But in the right-hand picture, the energy of the photon is larger (see the thicker line and bigger wiggles on the green arrow) so there is no allowed energy level for the excited electron to move to. Instead, the electron is kicked completely out of the material. To put that another way, the high-energy photons cause the material to emit electrons. This is photoemission!
The crucial part about ARPES is that the emitted electrons retain information about the quantum state that they were in before they absorbed the photons. In particular, the photons carry almost no momentum, so the momentum of the electron can’t really change during the emission process. But also, energy must be conserved, so the energy of the emitted electron must be the energy of the photon, plus the energy of the quantum state that the electron was in before emission.
So, if you can catch the emitted electrons, and measure their energy and momentum, then you can recover the band structure! The angle-resolved part in the ARPES acronym means that the momentum of the electrons is deduced from what angle they are emitted at.
But what does this look like in practise? Fortunately, a friendly guide from Diamond showed me around and let me take pictures.
The upper-left picture is an outside view of the Diamond facility. (The cover picture for this blog entry is an aerial view.) It’s a circular building, although this picture is taken from close enough that this might be hard to see. This gives a sense of scale for the place!
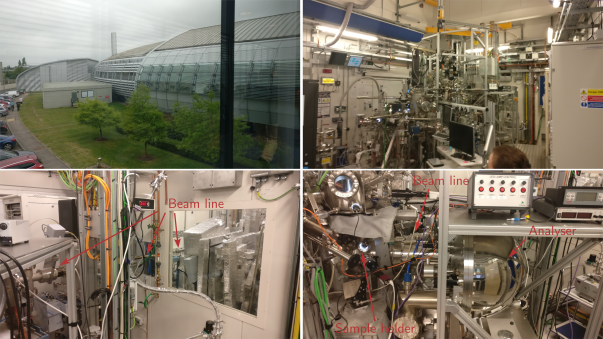
Inside is a machine called a synchrotron. They didn’t let us go near this, so I don’t have any pictures, but it is a circular particle accelerator which keeps bunches of electrons flowing around it very, very fast. As they go around, they release a lot of X-ray photons which can be captured and focused. (There is a really cool animation of this on their web site.) The X-rays come down a “beam line” and into one of many experimental “hutches” which stand around the outside of the accelerator.
The upper-right picture shows the ARPES machine inside the main hutch of beamline I05. Most of the stuff you can see at the front is designed for making samples under high vacuum, which can then be transferred straight into the sample chamber without exposure to air.
The lower-left picture is behind the machine, where the beam line comes in. It’s kinda hard to see the metal-coloured pipe, so I’ve drawn arrows. The lower-right picture shows where the real action happens. The sample chamber is near the bottom (there is a window above it which allows the experimentalists to visually check that the sample is okay), and you can just about see the beam line coming in from behind the rack in the foreground.
The X-rays come into the sample chamber from the beam line, strike the sample, and the emitted electrons are funnelled into the analyser which is the big metallic hemisphere towards the right of the picture. The spherical shape is important, because the momentum of the electrons is detected by how much they are deflected by a strong electric field inside the analyser. This separates the high momentum electrons from the low momentum ones in a similar way that a centrifuge separates heavy items from light ones.
And what can you get after all of this? The energy and momentum of all the electrons is recorded, and pretty graphs can be made!

Above is a picture that I stole from the Diamond web site. On the left is a theoretical calculation for the band structure of a material called tungsten diselenide (WSe2). On the right is the ARPES data. The colour scheme shows the intensity of the photoemitted electrons. As you can see, the prediction and data match very well. After all the effort of building a massive machine, it works! Hooray science!
